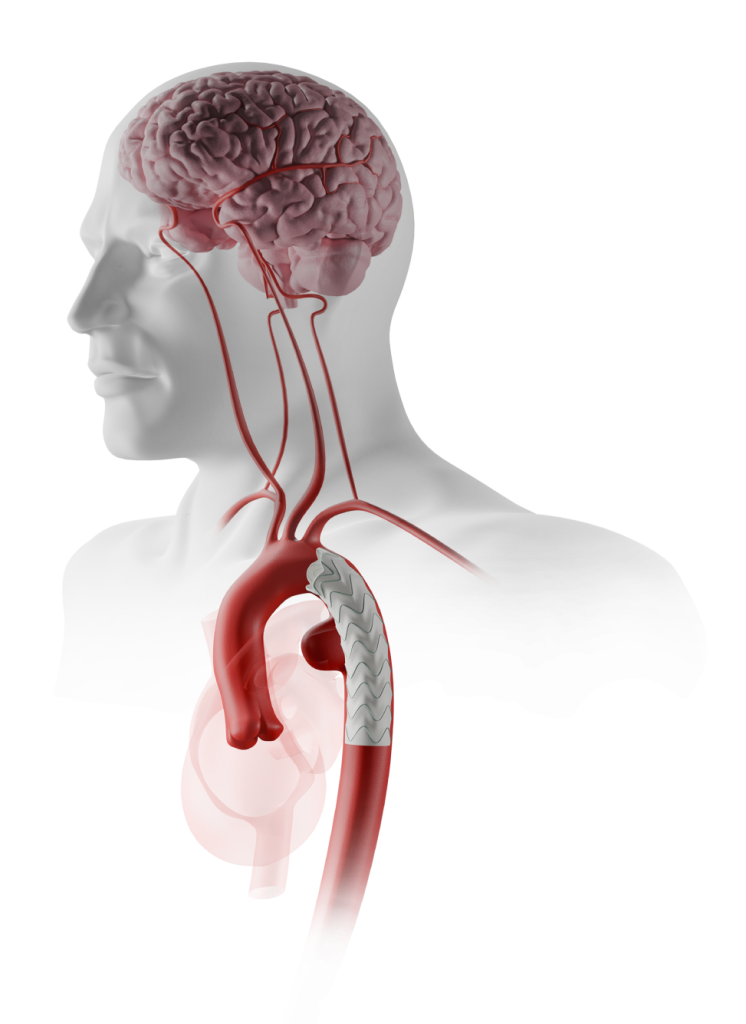
Neurological Events
Uniquely Inspired for Ideal Placement
Precision. Reliability. Outcomes.
Behind the Scenes of TEVAR: From Challenge...
Intraoperative solid or gaseous emboli are considered among the most common etiologies for NBI 1
Karaolanis et al. (2022)
NBI = Neurological Brain Injury
Potential NBI Risk Factors
Manipulation of Endovascular Instruments 1
Prothrombotic tendency of the wires and catheters 1
Deployment of large stent grafts in a diseased atherosclerotic aorta 1
CAN LEAD TO
Dislodgement of: 1
- Atheromatous debris
- Arterial Wall
- Fresh and organised thrombous
The publication and data presented here are provided as a general overview of TEVAR and may not directly relate to the approved indications for RelayPro. Indications for use may vary by region. Always consult the Instructions for Use for relevant indications, contraindications, warnings, and precautions.
Pooled prevalence of any stroke associated
with the TEVAR procedure 1
This meta-analysis included patients with a variety of thoracic aortic diseases treated with TEVAR, including Type B aortic dissection (37.5%), descending thoracic aortic aneurysm (33.6%), penetrating aortic ulcer (7.2%), pseudoaneurysm (6.1%), traumatic transection (5.3%), and other etiologies (10.3%) i.e., intramural hematoma, floating thrombus, septic conditions, endoleaks.
…to Impact
Short-term Impact 2
The development of perioperative stroke was associated with significantly higher: 2
Ullery et al. (2012)
Comparison Between Patients With and Without Perioperative Stroke
In Hospital Mortality
Arrythmia
Cardiac Arrest
In this retrospective review, indications for surgery among those patients developing perioperative stroke (3.8%, 20/530) included degenerative aneurysm (n=14), acute type B dissection (n=4), penetrating atherosclerotic ulcer (n=1), and aortic transection (n=1). The publications and data presented here are provided as a general overview on TEVAR and may not directly relate to the RelayPro approved indications. Indications for use will also differ by region. Always consult the Instructions for Use for the relevant indications, contraindications, warnings and precautions.
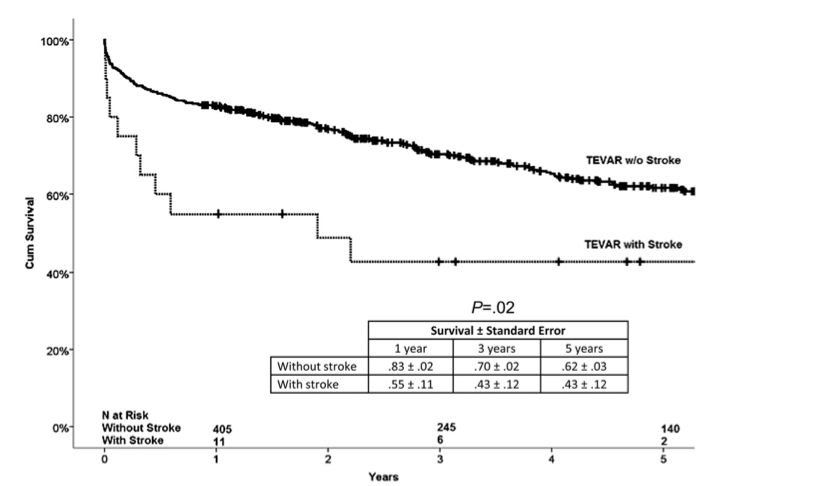
Long-term Impact 2
Patients without perioperative stroke had significantly better survival outcomes than those with perioperative stroke (log-rank, P=.02) 2
Ullery et al. (2012)
Kaplan-Meier survival outcomes for patients with and without perioperative strokes after thoracic endovascular repair (TEVAR)
In this retrospective review, indications for surgery among those patients developing perioperative stroke (3.8%, 20/530) included degenerative aneurysm (n=14), acute type B dissection (n=4), penetrating atherosclerotic ulcer (n=1), and aortic transection (n=1). The publications and data presented here are provided as a general overview on TEVAR and may not directly relate to the RelayPro approved indications. Indications for use will also differ by region. Always consult the Instructions for Use for the relevant indications, contraindications, warnings and precautions.
Discover the Truth, Experience the Difference: Relay®Pro
Stroke Rate through 1-Year Follow-up
 Terumo AorticRelay®Pro |  MedtronicValiant™ Captivia™ |  GoreTAG® Conformable |  CookZenith® | |
|---|---|---|---|---|
The data presented reflect cumulative results. Product images obtained from Medtronic, Gore and Cook, online. All rights belong to their respective owners. | ||||
Aneurysm and PAU | 3.6%(4/110) 3 | 6.5%(10/154) 4 | NA^ | 5%Zenith® Alpha™ |
Acute, Complicated Type-B Dissection | 1.8%*(1/56) 7 | 6%(3/50) 8 | NA^^ | 8.2%Zenith® TX2® |
Definition of stroke may differ by publication/study and may be differentiated by severity of outcome and/or timing post-index procedure. Additional details are provided within the relevant publications and should be considered. Stroke was adjudicated by the Clinical Events Committee per the specific study definition for references 3, 4, 5, 6, and 7. For the Cook dissection study, reference 10 and the Cook Zenith TX2 IFU (2022-05, I-DISSECT-SYSTEM-441-02EN) were used as references.
Stroke Rate through 1-Year Follow-up
 Terumo AorticRelay®Pro |
|---|
Aneurysm and PAU3.6%(4/110) 3 |
Acute, Complicated |
 MedtronicValiant™ Captivia™ |
|---|
Aneurysm and PAU6.5%(10/154) 4 |
Acute, Complicated |
 GoreTAG® Conformable |
|---|
Aneurysm and PAUNA^ |
Acute, Complicated |
 CookZenith® |
|---|
Aneurysm and PAU5%Zenith® Alpha™ |
Acute, Complicated |
The data presented reflect cumulative results. Product images obtained from Medtronic, Gore and Cook, online. All rights belong to their respective owners.
* Strokes which did not meet the study definition of disabling (a sudden, non-convulsive loss of neurological function due to an ischemic or haemorrhagic intracranial vascular event defined as focal neurological deficits that impair the subject’s day-to-day life as assessed by the CEC members, lasting for 365 days or longer) had a rate of 5.4% (3/56)
^ Gore IDE stroke rate data for the 1-year follow-up is not available. The only available stroke rate data for Aneurysm and PAU from the IDE study is at the 30-day follow-up, reporting a rate of 3% (2/66). 5
^^ Gore IDE stroke rate data for the 1-year follow-up is not available. The only available stroke rate data for Type- B Dissection from the IDE study is at the 30-day follow-up, reporting a rate of 18% (9/50) 9
Definition of stroke may differ by publication/study and may be differentiated by severity of outcome and/or timing post-index procedure. Additional details are provided within the relevant publications and should be considered. Stroke was adjudicated by the Clinical Events Committee per the specific study definition for references 3, 4, 5, 6, and 7. For the Cook dissection study, reference 10 and the Cook Zenith TX2 IFU (2022-05, I-DISSECT-SYSTEM-441-02EN) were used as references.
Relay®Pro Key Features
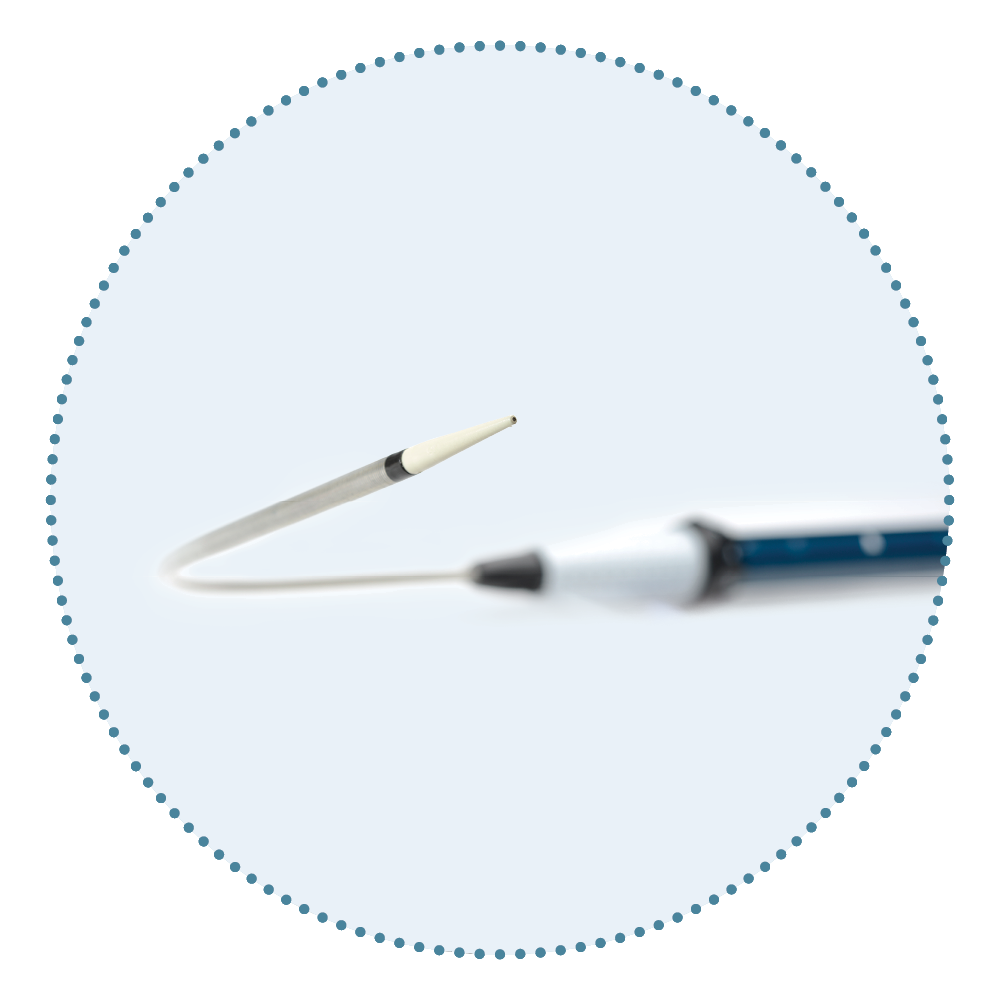
Low Profile Delivery System
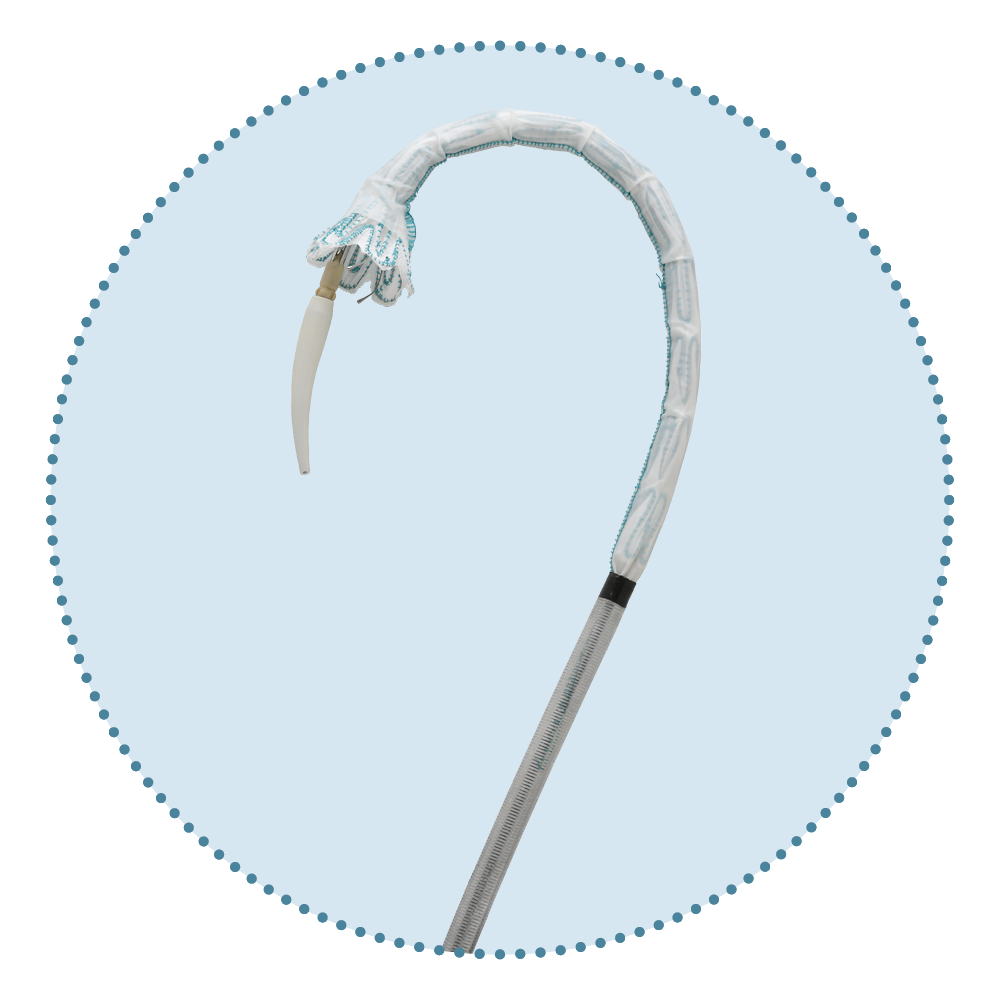
Dual Sheath Technology
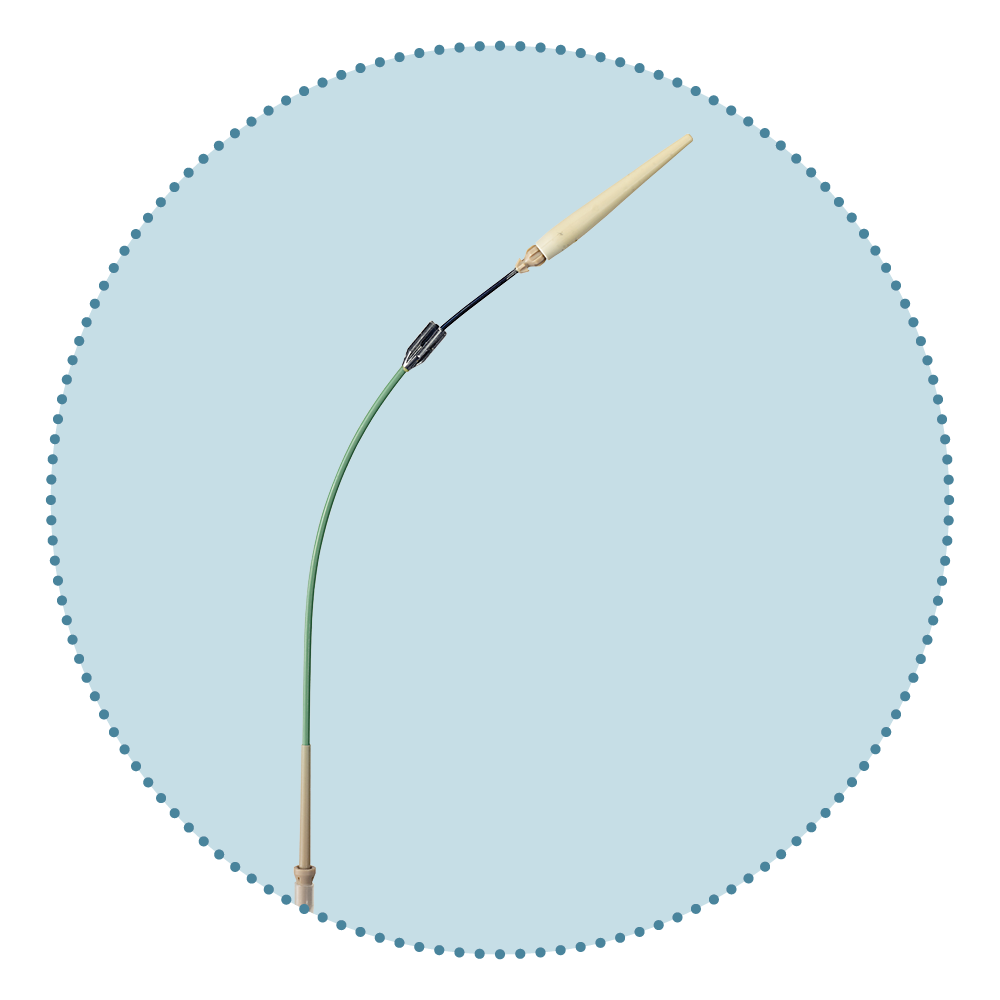
Pre-curved Nitinol Inner Catheter
…Low-Profile stent-grafts [LPSG**] might contribute to reducing embolization by less friction to the aortic wall during TEVAR compared with Conventional-sized stent-graft [CSSG] 11
Seike et al. (2023)
** Includes RelayPro and Cook Zenith Alpha
...one stable outer sheath for support during advancement and a second inner, flexible sheath which allows atraumatic navigation to and across the aortic arch 12
El Beyrouti et al. (2020)
The RelayPro delivery system design is independent of indication. The RelayPro is NOT indicated for erosion, rupture and the FDA-approved device is also NOT indicated for IMH and is only intended for the DTA. Approved indications will vary by region, always consult the corresponding IFU
...some devices were designed to reduce friction at the aortic arch by adding a curve to the device sheath 11
Seike et al. (2023)
Watch the Video
Neurological Events in TEVAR: Insights from Leading Physicians
The content, discussion and opinions in this video are those of the physician and may not be reflective of the approved indications for use of the device, which may also differ by region. Please consult eifu.terumoaortic.com for product indications, contraindications and warnings/precautions. Product availability subject to regulatory approval.
Republished with permission.
Downloads
Relay®Pro Features and Benefits.
Explore the features and benefits of RelayPro and how they contribute to the reduction of Neurological Events.
References
Karaolanis et al. (2022). A systematic review and meta-analysis of stroke rates in patients undergoing thoracic endovascular aortic repair for descending thoracic aortic aneurysm and type B dissection. Journal of Vascular Surgery
Ullery et al. (2012). Vascular distribution of stroke and its relationship to perioperative mortality and neurologic outcome after thoracic endovascular aortic repair. Journal of Vascular Surgery
Szeto et al. (2022). One-year results with a low-profile endograft in subjects with thoracic aortic aneurysm and ulcer pathologies. The Journal of Thoracic and Cardiovascular Surgery
Fairman et al. (2012). Pivotal results for the Medtronic Valiant Thoracic Stent Graft System in the VALOR II trial. Journal of Vascular Surgery
Jordan et al. (2015). Results of a prospective multicenter trial of CTAG thoracic endograft. Journal of Vascular Surgery
Illig et al. (2015). One-year outcomes from the international multicenter study of the Zenith Alpha Thoracic Endovascular Graft for thoracic endovascular repair. Journal of Vascular Surgery
Rossi et al. (2024). One-Year Results of a Low-Profile Endograft in Acute, Complicated Type B Aortic Dissection. The Annals of Thoracic Surgery
Bavaria et al. (2022). Five-year outcomes of endovascular repair of complicated acute type B aortic dissections. The Journal of Thoracic and Cardiovascular Surgery
Cambria et al. (2015). Multicenter clinical trial of the conformable stent graft for the treatment of acute, complicated type B dissection. Journal of Vascular Surgery
Lombardi et al. (2022). Five-year results of the STABLE II study for the endovascular treatment of complicated, acute type B aortic dissection with a composite device design. Journal of Vascular Surgery
Seike et al. (2023). Lower-profile stent graft reduces the risk of embolism during thoracic endovascular aortic repair in shaggy aorta. Interdiscip Cardiovasc Thorac Surg
El Beyrouti et al. (2020). Early results of a low-profile stent-graft for thoracic endovascular aortic repair. PLOS One
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
