TREO®
Versatile by Design.
Fit for Any Anatomy.*
Repair. Remodel. Results.
*Per IFU
A Strong Foundation of Evidence...
Studies show early, and significant sac regression is linked to improved survival and fewer late complications 1,2,3
The amount of sac regression is associated with a lower risk of long-term mortality, reinterventions, and ruptures. 2
Vinamr Rastogi, et al
Patients with sac regression of >5 mm and no endoleaks in the 2-year CTA have a 95.6% probability of reintervention-free outcomes. 3
Suvi J. Väärämäki, et al
TREO®
Sac Regression at 2 Years 4
63/116
Absence of Type I Endoleak at 2 Years 4
112/113
Absence of Reinterventions at 2 years 4
129/132
Type III & IV Endoleak at 2 Years 4
0/113
TREO®'s Positive Durable Outcomes Through 5Y 5
(0/150)
- Rupture
- Aneurysm Related Mortality
- Conversion to Open Repair
TREO® demonstrates consistent sac diameter regression
Average Sac Diameter Reduction in IDE sub-cohort
13mm
TREO® IDE 5 and PAS *6 Aneurysm Sac Average Maximum Diameter
Subjects w/ Decrease >5 mm at 2 years
(CORE LABORATORY REPORTED)
Average Sac Diameter Reduction in PAS sub-cohort
13mm
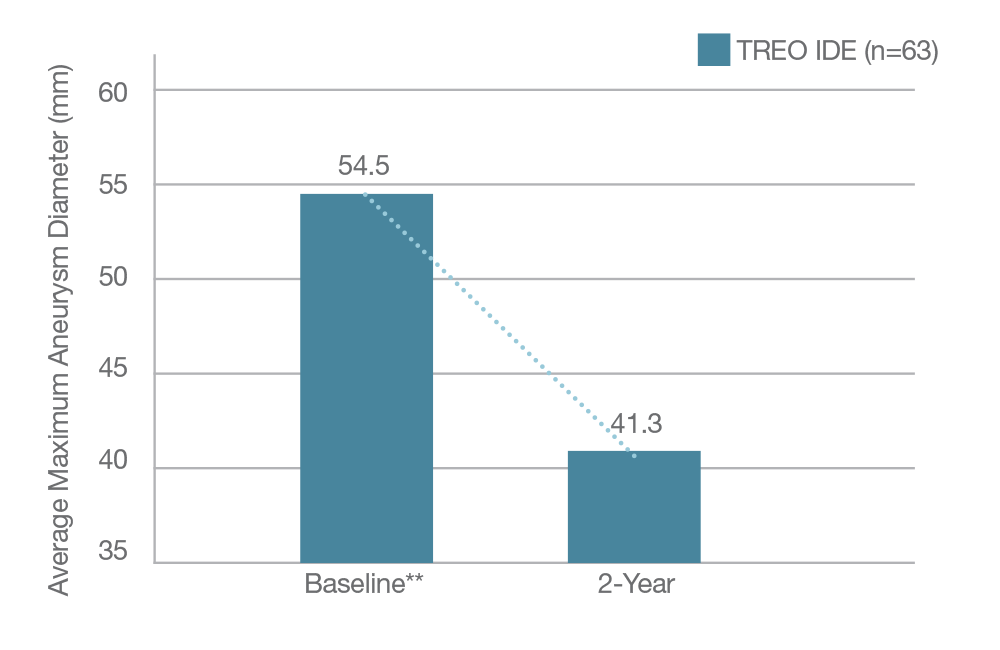
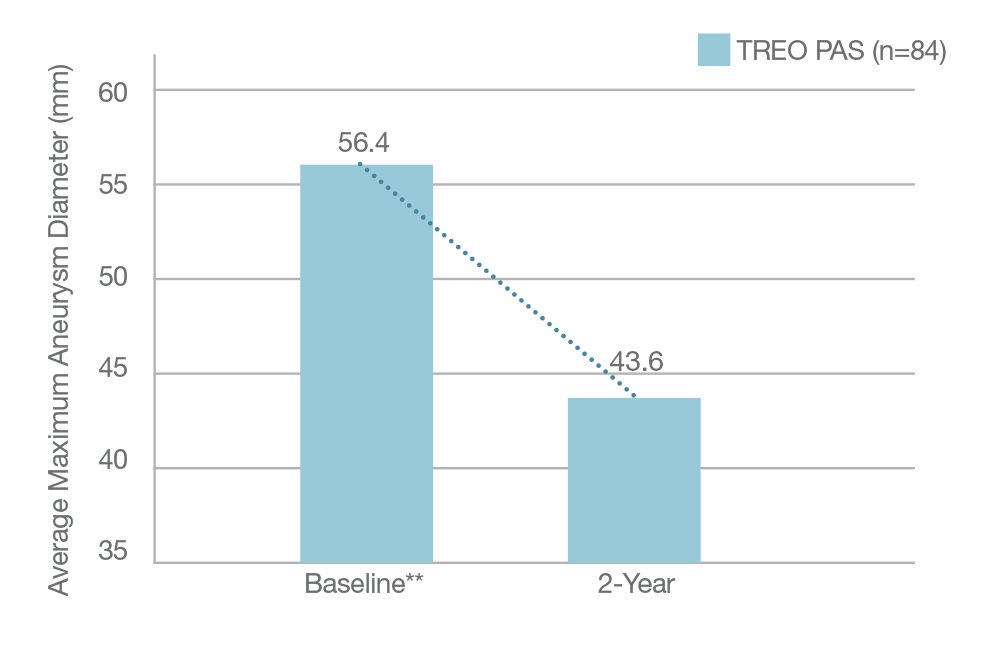
* The US Post Approval Study is in active follow-up; data in the chart is taken from October 2024.
** 30 day imaging
TREO®’s Sac Regression Results are a Reflection of its Design
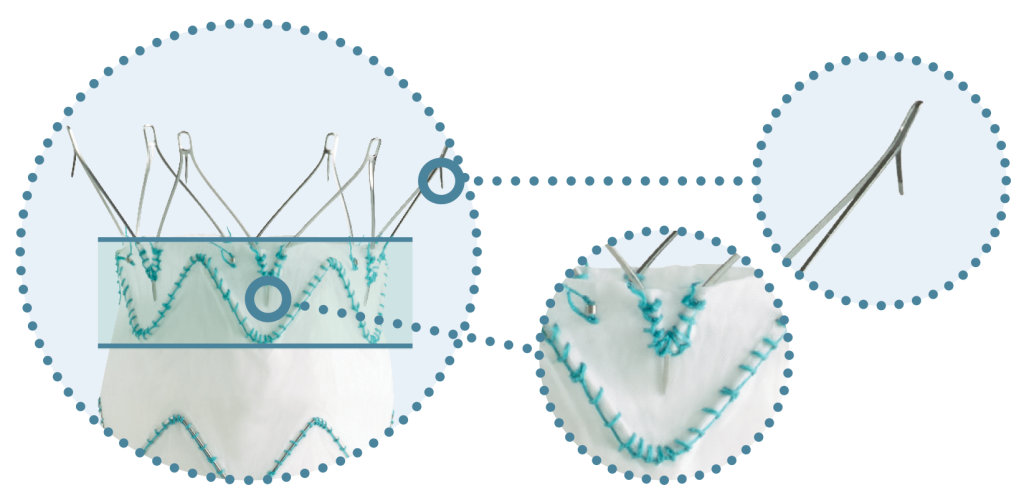
Dual Active Fixation & Optimised Proximal Seal
Migration through 5 years 5
1/150
Type Ia Endoleak through 5 years 5
4/150
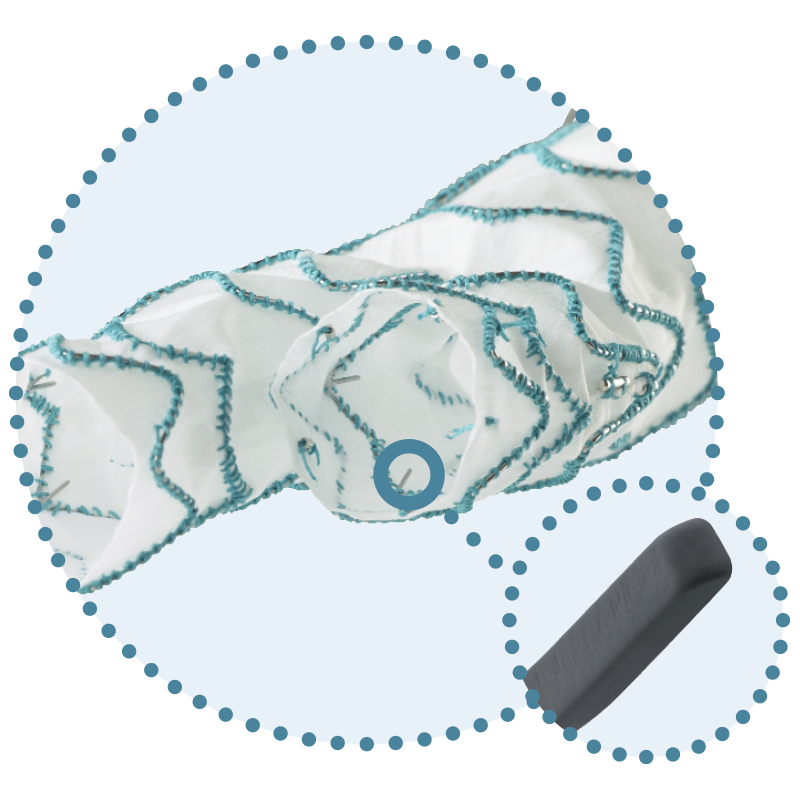
Lock Stent Technology
Type III EL through 5 years 5
0/150

Next Generation Polyester Design with Long Main Bodies
Type IV EL through 5 years 5
0/150
80, 100, 120mm
High quality clinical data shows consistent rates of sac regression and low sac expansion in TREO®
TREO® Aneurysm sac changes @ 1 year and 5 years
Clinical Study | IDE5 (1&5 Years) | Feasibility Study 7 (1&5 Years) | RATIONALE * 8 (1 Year) | US PAS ^ 6 (1 Year) | Marone ** 9 (5 Years) |
|---|---|---|---|---|---|
^ TREO US PAS is an all-comers study, follow-up on-going | 73% of Patients have Hostile Neck Anatomy 9 27/37 | ||||
Number of Patients | 136 | 28 | 202 | 226 | 31 |
Decrease @ 1 Year | 46% | 54% | 54% | 46% | 71% |
Stable @ 1 Year | 54% | 46% | 43% | 50% | 29% |
Increase @ 1 Year | 0% | 0% | 3% | 4% | 0% |
5 Year Decrease | 61% 43/70 | 81% 17/21 | N/A | N/A | 71% |
TREO®’s Design & Sac Regression Success
- 32% aneurysm size reduction (23mm) at 1 Year
- Without usage of adjunctive devices
Baseline and 1-Year Results
Before
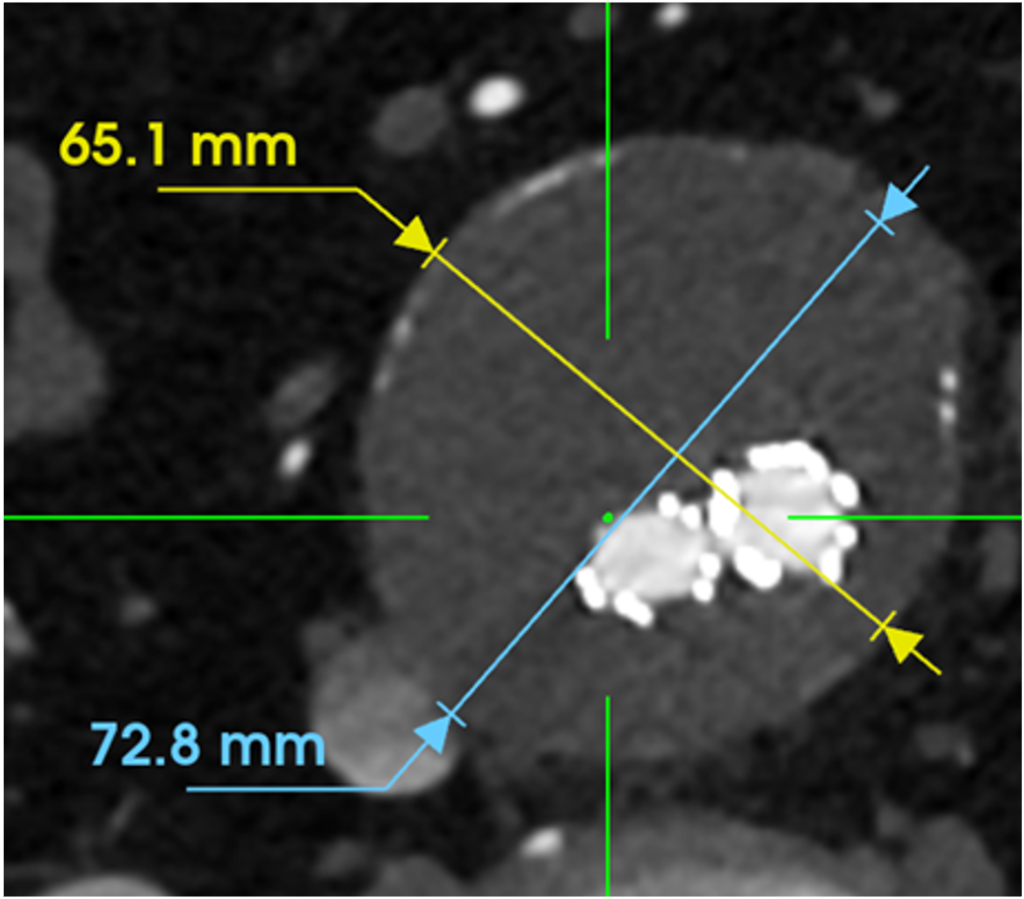
After
Case images courtesy of Dr. Jonathon Rollo.
Sac Regression: The Ultimate Indicator of EVAR Success.
Discover more about TREO®s features and benefits and find out how they contribute to Sac Regression and Long term AAA results in aortic remodeling with aneurysm regression.
Features & Benefits
TREO®
Discover how each of the key features and benefits are integrated into TREO to ensure the highest quality and performance possible.

References
Rastogi, Vinamr et al. “One year aneurysm sac dynamics are associated with reinterventions and rupture following infrarenal endovascular aneurysm repair.” Society of Vascular Surgery, volume 79, number 2, pp 269-279.
Antoniou, George A. et al. “Prognostic Significance of Aneurysm Sac Shrinkage After Endovascular Aneurysm Repair.” Journal of Endovascular Therapy, Vol 27 (5), 2020, pp 857-868. https://doi. org/10.1177/1526602820937432.
Väärämäki, Suvi et al. “Possible implications of device-specific variability in post-endovascular aneurysm repair sac regression and endoleaks for surveillance categorization.” Journal of Vascular Surgery, Vol 78, (5), November 2023, pp 1204-1211. https://doi.org/10.1016/j.jvs.2023.07.001
TREO US IFU
IDE 5-year FDA Report 2024, data on file.
US Post Approval Study Follow-up to Oct. 2024, data on file.
Terumo Aortic TREO Annual Report 100200 2019 data on file.
Uberoi et al. “Global Post-Market Clinical Follow-up of the Treovance Stent-Graft for Endovascular Aneurysm Repair: One-Year Results From the RATIONALE Registry.” Journal of Endovascular Therapy vol. 25, 6 (2018): pp. 726-734. doi:10.1177/1526602818803939
Marone et al. “Five-Year Outcomes of Endovascular Aortic Repair With the TREO Abdominal Endograft.” Journal of Endovascular Therapy vol. 0, 0 (2023). doi:10.1177/15266028231170161
Product Disclaimer
Product availability subject to regulatory approval.
An EU Declaration of Conformity may be requested from regulatoryaffairsuk@terumoaortic.com
Instructions for Use
View the eIFU for more information on use, indications, contraindications, warnings/precautions and availability within your market.
